
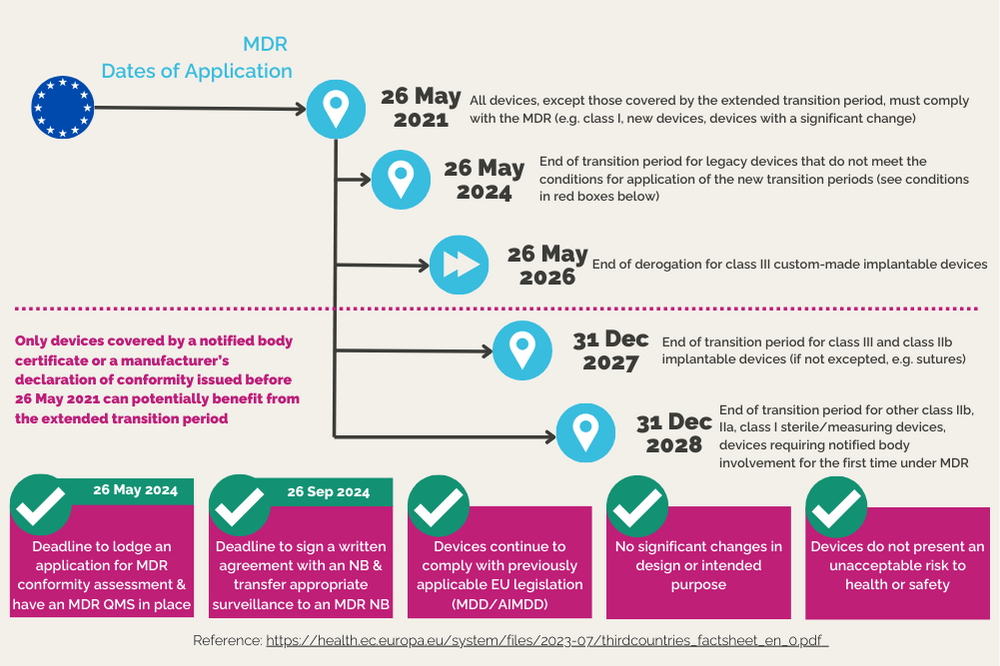
Certain substance-based medical devices that are not yet certified under the MDR may retain their market authorization under specific conditions until 31 December 2027, or even until 31 December 2028 (see the extended transition period defined in Article 120 of Regulation (EU) 2017/745 on medical devices (MDR)).
However, in order to achieve this, the following important upcoming deadlines must be met:
- 26 May 2024:
- Manufacturers need to have a QMS system in accordance with MDR requirements in place and must submit a formal application for conformity assessment to a Notified Body by 26 May 2024.
- 26 September 2024:
- Manufacturers need to have a signed contract with a Notified Body.
There is still time to get in touch with a Notified Body and prepare the necessary documents. We will be happy to assist you in all aspects of keeping your MD on the market.
If we could support your company in this case, please contact us.
